–
Description
Orcein was originally obtained from lichens, but is now made from orcinol by hydrogen peroxide oxidation with ammonia present. It is a standard dye to use for the demonstration of elastic fibres, when it is applied in an acid, alcoholic solution. This same solution has also been shown to stain the rough endoplasmic reticulum of hepatitis B infected liver cells (ground glass cells).
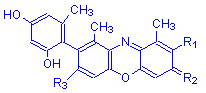
Orcein is a variable mixture of several compounds. These compounds differ at the points marked R1, R2 and R3 in the structural formula. The chart below gives the commonest eight of these compounds, with the groups at each of the three points. The % column is the approximate percentage of each compound in synthetic orcein.
| Compound | R1 | R2 | R3 | % |
|---|---|---|---|---|
α-Aminoorcein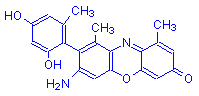 | H | O | NH2 | 10 |
α-Hydroxyorcein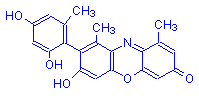 | H | O | OH | 10 |
β-Aminoorcein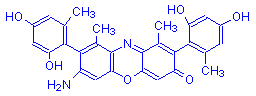 | Orcinol | O | NH2 | 6 |
γ-Aminoorcein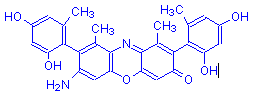 | Orcinol | O | NH2 | 12 |
β-Hydroxyorcein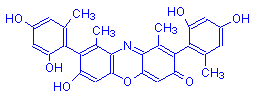 | Orcinol | O | OH | 15 |
γ-Hydroxyorcein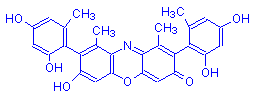 | Orcinol | O | OH | 10 |
β-Aminoorceimine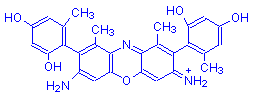 | Orcinol | NH | NH2 | 1-2 |
γ-Aminoorceimine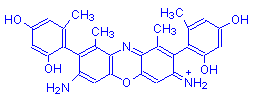 | Orcinol | NH | NH2 | 1-2 |
References
- R. D. Lillie.
Conn’s Biological Stains
Williams & Wilkins, Baltimore, MD., U.S.A. - Aldrich chemical catalogue, 1992
Aldrich Chemical Company, Milwaukee, WI, USA. - Susan Budavari, Editor,
The Merck Index, Ed. 12
Merck & Co., Inc., Whitehouse Station, NJ, USA






