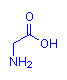
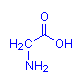
Amino acids are so called because their molecules have both a (basic) amino group and an (acid) carboxyl group present. The sole exception to this is proline. In the case of alpha amino acids, these two groups are attached to the same carbon atom – designated to be carbon number 1 (or alpha, α – this being the name of the first letter in the Greek alphabet). This is illustrated below with the simplest amino acid, glycine. The leftmost diagram follows the standard convention. That on the right explicitly shows the alpha carbon which is assumed in the first diagram. All alpha amino acids have this arrangement at one end of their molecule.
Apart from these amino and carboxyl groups, amino acids may have other groups attached at various points. These often include the ubiquitous methyl group, but may also include reactive groups such as additional amino, carboxyl, and hydroxyl groups, sulphur and various rings. These are the side groups, which are often mentioned. A point to note is that these side groups are the ones that ionize and react with dyes. The amino and carboxyl groups that give the compounds their name are not generally available. Amino acids may be classified according to these side groups:
No Reactive Side Groups

Glycine
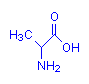
Alanine
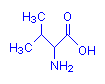
Valine
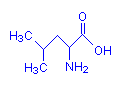
Leucine
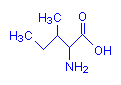
Isoleucine
Containing Hydroxyl Groups
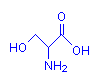
Serine
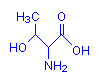
Threonine
Containing Sulphur
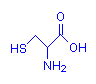
Cysteine
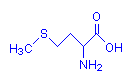
Methionine
Containing Additional Amino Groups
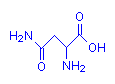
Asparagine
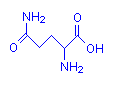
Glutamine
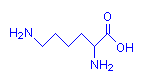
Lysine
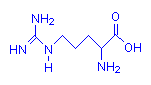
Arginine
Containing Additional Carboxyl Groups
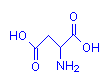
Aspartic acid
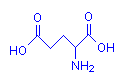
Glutamic acid
Containing Rings
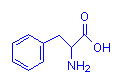
Phenylalanine
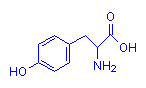
Tyrosine
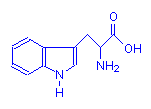
Tryptophan
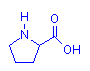
Proline
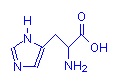
Histidine