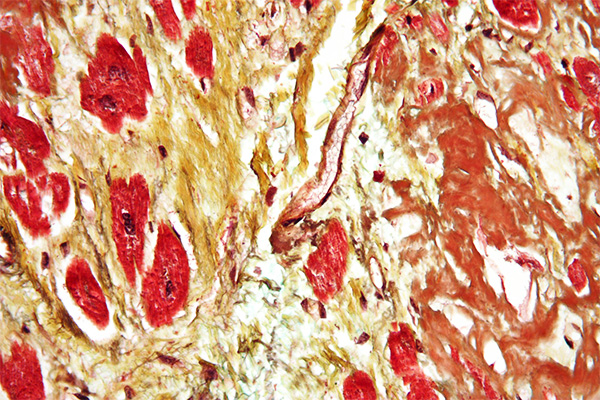
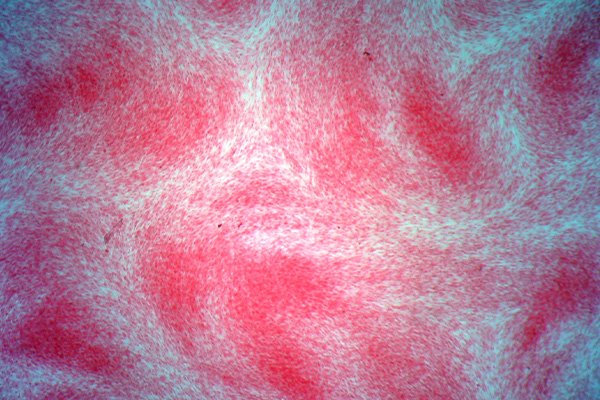
Elastic is the name given to some fibers that are widely distributed throughout the body, such as skin, arteries and veins, lung, etc. Generally, they are found where some movement is required from the tissue involved and, as the name implies, they help restore the tissue after movement. They may be increased or decreased in amount due to disease and it may be necessary to demonstrate them for that reason, but their demonstration is quite often used to show that a structure is a blood vessel, since both arteries and veins have a ring of elastic fiber in their wall.
Sometimes the word “elastin” is used for these fibers. Strictly speaking this is incorrect as elastin is the name of a major protein component of elastic. The fibers themselves are referred to as “elastic fibers”.
Introduction
Elastic fibers are resistant to autolysis and are quite easily fixed. Most fixatives preserve them, and standard formalin variants work well. Under ultra-violet light they may have a green to blue-white fluorescence, the color depending on the particular filters used. The coarser fibers can often be seen in H&E stained sections as a smooth, pale pink material, but fine fibers are much more difficult or impossible to see and for their unequivocal identification selective staining methods are necessary. They are not infrequently seen in sections stained for amyloid with direct cotton dyes, such as congo red and sirius red F3B. This is likely due to both amyloid and elastic fibers being stained via non-polar forces.
Methods for the demonstration of elastic fibers are of several types, listed below. Most of these methods are satisfactory in practice. However, it should be noted that elastic fibers vary in their size and thickness. Large arteries such as the aorta, for instance, may have very coarse fibers of quite thick elastic in the arterial wall, whereas the tissue surrounding the alveoli in the lung may have quite small, fine fibers. This physical difference can affect the choice of methods used to demonstrate the fibers.
There has been an evolution in the explanations for the selectivity of methods for elastic. It used to be explained on the basis of ionic staining, and those methods which overstained were differentiated to remove dye from the less densely stained tissue. Then it was realized that non-polar forces played a part and the explanation was based on hydrogen bonds. It was then shown that the fibers still stained when some dyes not capable of forming hydrogen bonds were used. However, these dyes all had multiple aromatic rings and were all capable of bonding by Van der Waal’s forces. That is now the most commonly given explanation, that dyes bind preferentially to elastic fibers due to van der Waal’s forces. The explanation appears to be valid for all the types of methods.
Elastic Fiber Types
Oxytalan Fibers
Oxytalan fibers are thought to be immature elastic fibers. After oxidation with peracetic acid, performic acid or potassium pemanganate they may be demonstrated by aldehyde fuchsin, but reportedly do not stain with Verhöeff’s hematoxylin. They are defined as a type of connective tissue fiber representing an early stage in the formation of elastin fibers and found throughout the body, particularly in the periodontal ligament and gingivae.
Elaunin Fibers
Elaunin fibers are defined in the same dictionary as a component of elastic fibers formed from a deposition of elastin between oxytalan fibers; found in the periodontal ligament and in the connective tissue of the dermis, particularly in association with sweat glands.
Bancroft notes that elaunin fibers may be stained with orcein, aldehyde fuchsin and iron-resorcin-fuchsin, but do not stain with Verhöeff’s hematoxylin nor with orcinol-new fuchsin.
Orcein Dye Staining of Elastic Fibers
Orcein is a complex mixture of related compounds originally derived from lichens but now made synthetically. The synthetic product is often considered to be superior in staining abilities to the natural product and is invariably the material provided from laboratory supply companies. It was one of the first dyes used to selectively stain elastic fibers, and demonstrates both thick and thin fibers in a single section. The simplest technique is likely that of Taenzer-Unna, which uses a 1% orcein solution in 1% acid alcohol for an appropriate time, recommendations varying from a half hour to overnight. This same method is also used to stain the rough endoplasmic reticulum in ground glass cells in liver biopsies. Once stained, orcein is fairly resistant to extraction and other techniques can be applied to differentiate tissue constituents.
Orcein staining of elastic fibers has frequently been incorporated into other staining techniques, notably the trichrome methods, the combinations usually being referred to as pentachrome stains. These methods may stain elastic fibers as a separate step, or may incorporate orcein into a staining solution to accomplish the same thing. Such methods are often based on Unna’s technique which used the orcein in a solution with aniline blue or one of its constituent dyes, but add other dyes to the staining mix to differentiate between collagen and other structures more effectively.
Iron Resorcin Fuchsin Staining of Elastic Fibers
The iron-resorcin-fuchsin variants, of which there are several, are quite popular for the demonstration of elastic fibers. Usually, the results are well stained elastic fibers of all thicknesses in a single section. Weigert’s original technique used basic fuchsin, but several other dyes, alone and in combination, have also been shown to give effective staining in different colors which make the fibers more prominent. Often, depending on the method, a Mallory bleach may be required before the staining solution is applied.
Black, or near black staining of elastic fibers is often considered desirable, either because it is reminiscent of Verhöeff’s iron hematoxylin technique, which is so popular, or because it contrasts so well with many counterstains. Miller’s variant produces black stained fibers with overnight staining and Humberstone’s produces blue-black fibers, and may be somewhat faster with an older solution.
Most of these methods use resorcinol but at least one uses orcinol. Orcinol is the compound used as the starting point for making synthetic orcein. It is also worth noting the relationship these compounds have with phenol, as at least one method incorporates it into the staining solution.
Resorcinol
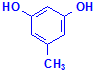
Orcinol
Phenol
The compounding procedure for iron-resorcin-dye solutions is to combine the dye with the resorcinol, bring to the boil, then add ferric chloride. A precipitate of unspecified composition forms which is presumed to be a complex of the resorcinol and dye, possibly with iron present. The precipitate is collected by filtration, dried, then redissolved in ethanol, often with some acid added. Depending on the dye or dye combinations used, this solution is stable from a few months to several years.
Some variants replace part of the dye content, or add to it, by incorporating dextrin into the solution. Dextrins are partially hydrolyzed starch with varying numbers of glucose units. They are probably incorporated into the compound which is precipitated along with the resorcinol and dyes, but the composition is unspecified again. It is presumed that the dye, the resorcinol and dextrin, if added, combine to form large molecules which are precipitated by the ferric chloride, possibly with some chemical attachment by the iron. The large molecules formed are prone to van der Waal’s forces and attach to the elastic fibers by them.
Ferric chloride is available in two solid forms, as an anhydrous powder and as hydrated crystals (FeCl3.6H20). Usually, the ferric chloride used to make the iron-resorcin-dye precipitates is in the form of a 30% solution of hydrated crystals. Occasionally, however, some other concentration is specified, so the directions should be read carefully (compare the orcinol-new fuchsin variant to Humberstone’s).
These methods have often been considered tedious to use, since the preparation takes some time. It involves the formation of the dyeing complex, its precipitation, collecting and drying, redissolving it in ethanol with heat, then adding acid. This often takes two days, and the early solutions had a life of only a few months, so the procedure had to be repeated fairly often. Some of the more modern variants are much less tedious to use as they are stable for several years and can therefore be made in larger quantities with the expectation that they will be sufficient for some year’s use. The Humberstone’s variant is particularly recommended. It has been used when it is in excess of ten years old with perfectly satisfactory results. Although the authors say that buffered formalin fixation is not recommended, the present system of short fixation does not appear to inhibit the staining at all.
Aldehyde Fuchsin Staining of Elastic Fibers
Aldehyde fuchsin stains several different tissue constituents, among them elastic fibers. They stain deep purple on a light purple background, usually, and are quite easy to see. It is suspected that the reason for the method not being used as much as it could be, has more to do with the poor keeping qualities of the staining solution than the quality of the results. The solution is stable for just a few months, so must be frequently prepared.
Aldehydes and basic fuchsin can form large molecules in solution and, once again, these may be prone to forming bonds via van der Waals forces, as far as elastic fibers are concerned, although other processes may be involved with other tissue constituents that aldehyde fuchsin colors.
Iron Hematoxylin Staining of Elastic Fibers
Verhöeff’s method, which is one of the most popular techniques, requires that attention be focused on one type of fiber at the expense of the others as it cannot demonstrate the coarsest and finest fibers at the same time, requiring a choice as to which are to be optimally stained. Fortunately, in most practical applications, the elastic fibers in any single section are usually similar in size and the staining of each section may be optimized for the fibers it contains. Should it be necessary to demonstrate thick and thin fibers in the same section then a method other than Verhöeff’s may be more satisfactory.
Verhöeff’s method has become popular because it is fairly rapid, taking perhaps 60-90 minutes to perform. The elastic fibers are darkly stained (black) and stand out well in contrast to other tissues, which may be yellow-brown or red from a Van Gieson counterstain. It also photographs well. Its major disadvantage is that it is not an automatic method, that is, its success depends on the skill of the person performing the technique, although that is not difficult to do.
Musto’s method is a modification of Verhöeff’s, but stains from a low pH solution containing hydrochloric acid. This makes the solution progressive, and differentiation of the elastic fibers is not required. Musto recommended a modification of the HPS method, but replaced phloxine with woodstain scarlet. A variation using a Van Gieson counterstain was also given.
Musto’s approach is not unusual, as several methods incorporating elastic fiber stains, often based on Verhöeff’s hematoxylin counterstained with HPS or other trichrome variants, have been suggested under the general term of pentachrome or Movat methods, some with and some without mucin staining. Fundamentally, they are an elastic fiber stain with a trichrome counterstain of some kind, either of the Masson type or of the HPS type. The Silverman-Movat technique is similar but uses orcein to stain the elastic.
Miscellaneous Methods
There are a few other methods for staining elastic fibers, although they are not in common use. One uses alum hematoxylin buffered to a pH of around 8.0, when the hematoxylin is selective for elastic. Another uses an unusual dye, alkali blue, which includes a quite rare example of afterchrome mordanting, i.e. methods in which the dye is applied first and when the element is stained, the mordant is applied. A third is a very quick technique using the Brown & Brenn counterstain which demonstrates elastic as red fibers on a yellow background in just a few minutes. None of these three methods are mainstream.
References
- Baker, John R., (1958)
Principles of biological microtechnique
Methuen, London, UK. - Bancroft, J.D. and Stevens A. (1982)
Theory and practice of histological techniques, Ed. 2
Churchill Livingstone, Edinburgh & London, UK.