Nucleic acids are compounds formed from the two pentose sugars ribose and deoxyribose. Deoxyribose has one less hydroxyl group than ribose, with hydrogen replacing it, i.e. it has lost one oxygen, hence the name. This is shown in the diagrams below. The top line shows ribose and the bottom line shows deoxyribose. The formulas to the right show the hydrogen atoms to emphasize the oxygen which has been removed in deoxyribose at carbon 2. The numbering scheme is shown to the left.
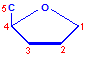
Numbering
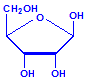
Ribose
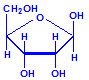
Ribose, explicit
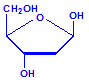
Deoxyribose
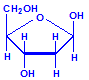
Deoxyribose, explicit
Ribose and deoxyribose are the basis of RNA (ribosenucleic acid) and DNA (deoxyribosenucleic acid) respectively, although they are also important as a source of energy and participation in cellular respiration. The underlying structure is the formation of a nucleoside. Nucleosides are compounds formed between one of the sugars and one of five bases, shown below as being in two groups, either one of two purines or one of three pyrimadines.
Purines
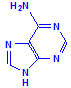
Adenine
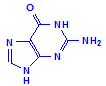
Guanine
Pyrimadines
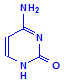
Cytosine
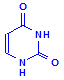
Uracil
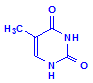
Thymine
Each of the pentoses can combine with the bases to form different nucleosides, illustrated below with the nucleoside adenosine and its complement cytidine. All of the nucleosides are formed similarly, with adenine giving rise to adenosine, guanine to guanosine, cytosine to cytidine, uracil to uridine and thymine to 5-methyluridine. Similar nucleosides form with deoxyribose.
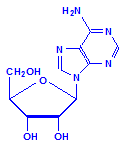
Adenosine
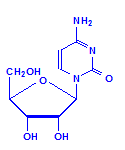
Cytidine
Nucleotides differ from nucleosides in that they have phosphate groups attached. This is commonly at carbon 5, where it says CH2OH in the formulas above. More than one phosphate group can be attached so that we could have adenosine monophosphate, adenosine diphosphate and adenosine triphosphate, for example. That last, adenosine triphosphate, is also referred to as ATP and is an important compound for the provision of energy in cellular metabolism. Other nucleotides are formed from nucleosides of both ribose and deoxyribose in similar fashion.
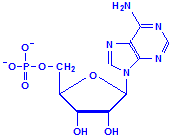
Adenosine monophosphate
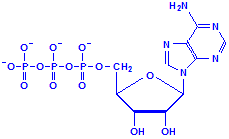
Adenosine triphosphate, ATP
The nucleic acids are composed of multiple units of nucleotides, linked through their phosphate groups, i.e. they are polymers, sometimes very long ones composed of millions of base pairs. They exist as a helical double strand, similar to a ladder with the bases as the rungs and the phosphate groups as the uprights. The double strands come about because the bases of the nucleotides pair up with each other, each linking to a specific, complementary base. In both DNA and RNA, guanine always pairs with cytosine, while in DNA, adenine pairs with thymine, and in RNA, adenine pairs with uracil. The link between the bases is due to hydrogen bonds. It is the sequence of bases that determines the “meaning” in the nucleic acids.
It would be very tedious to draw structural formulas of a nucleic acid sequence, so a shorthand system has been described. The bases are each assigned a letter and these letters are given as the sequence. It is to be understood that each base a letter represents is attached to either ribose or deoxyribose as the case may be, and the complementary base is attached to the one specified. Certain dyes can bind to DNA, such as 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) which binds to AT-rich regions of double-stranded DNA, fluorescing blue and often being used as a counterstain to detect nuclei in multicolor fluorescence applications.
| Base | Symbol | Complementary Base | ||||
|---|---|---|---|---|---|---|
| RNA | DNA | |||||
| Adenine | A | U | T | |||
| Cytosine | C | G | G | |||
| Guanine | G | C | C | |||
| Thymine | T | – | A | |||
| Uracil | U | A | – | |||
The three major components of nucleic acids, i.e., pentose sugars, phosphate and bases, are all important from a histological perspective. The pentose sugars may be used to specifically demonstrate DNA by acid hydrolysis of deoxyribose to form an aldehyde that reacts with Schiff’s reagent and colors it red. The phosphate groups have been shown to be involved in nuclear staining with hemalum and basic dyes, and the bases may be involved with the attachment of acid dyes as well as being capable of hydrogen bonding which, along with van der Waal’s forces, is known to be a factor in nuclear staining.
Although making up a large part of the nucleus, nucleic acids are not the only component. There is also some protein associated with it. This is referred to as nucleoprotein, and it may also be involved in nuclear staining. This protein includes histones, which are strongly alkaline proteins closely associated with DNA. There may be other, non-histone proteins present as well. DNA-histone complexes are now referred to as chromatin. This term originally came into use in the late 1800s before the structure of DNA was known and it was used to refer to that part of the nucleus that stained with dyes. It is now used to refer to the DNA-histone complex. Nevertheless, the word “chromatin” may still be encountered in contexts that refer to either of the meanings, particularly in older texts.
Nuclear staining may therefore be due to:
- Deoxyribose aldehyde
- Basic dyes attaching to phosphate groups
- Lakes attaching to phosphate groups
- Basic dyes attaching to nuclear protein
- Acid dyes attaching to nuclear protein
- Acid dyes attaching to nuclear basic groups
- Hydrogen bonding from bases
- Hydrogen bonding from other sources
- van der Waal’s forces
It is this great diversity of causes which explains why nuclear structure can be demonstrated after nucleic acids have been removed from tissue, whether deliberately or from leaving in a decalcifying acid for far too long, and why acid dyes have been used to stain nuclei in some older trichrome techniques. Unfortunately, determining which of these factors is at play in a particular staining method is not a simple matter. The reality is that it may not be a simple either/or explanation and multiple factors may be involved, as appears to be the case with alum hematoxylin staining of nuclei.