Immunostaining Cell Markers in PSC-Derived Kidney Organoids
This protocol is for fixing and immunostaining pluripotent stem cells (PSC)-derived kidney organoids in one well of a 96-well plate. If using other cultureware, adjust volumes accordingly. For this protocol, the organoids were generated using the STEMdiff™ Kidney Organoid Kit.
Expected Results
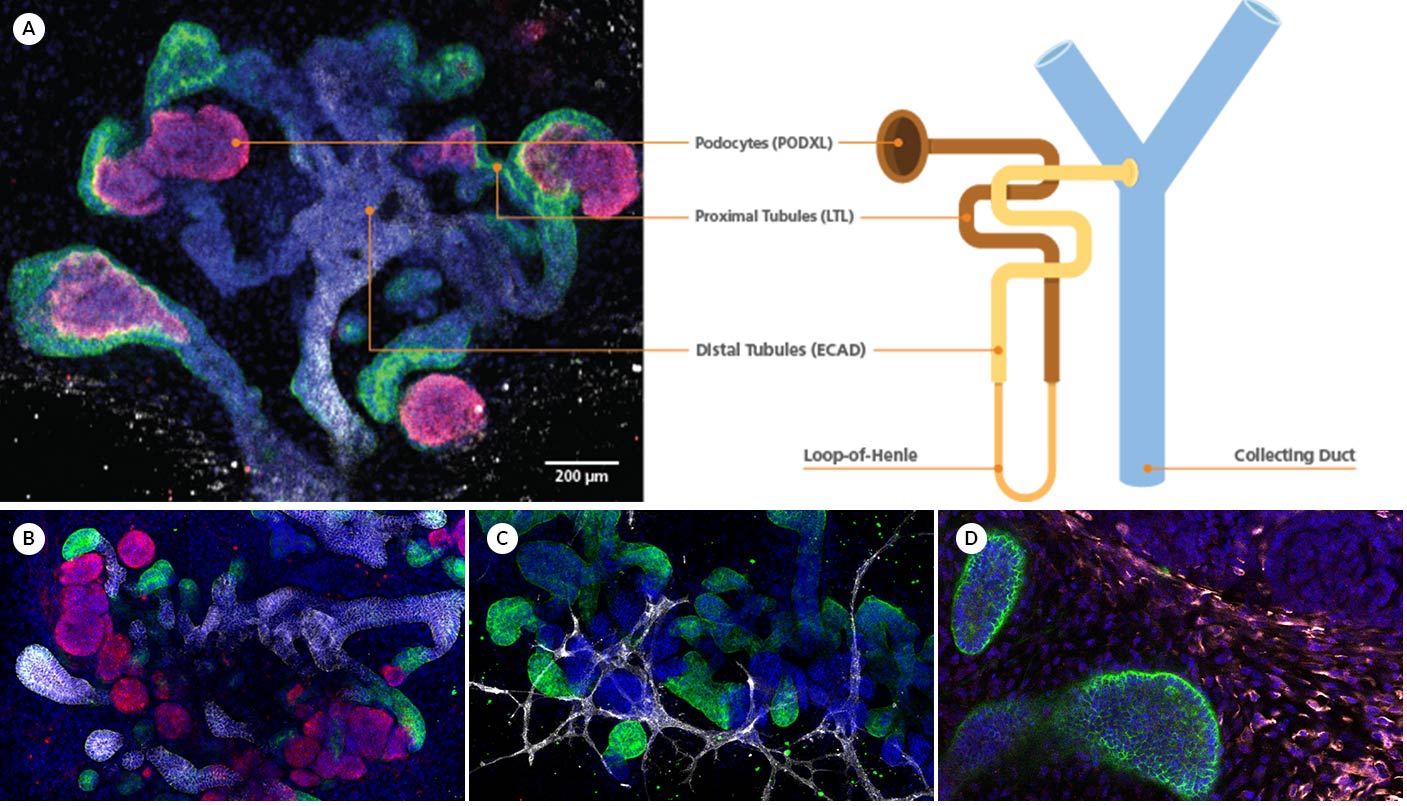
Figure 1. Immunofluorescence for Podocalyxin (PODXL), LTL, E-cadherin (ECAD), CD31, Vimentin (VIM), and MEIS1/2/3 in Kidney Organoids
(A) During differentiation, kidney organoids form convoluted tubular structures that resemble the structure and segmentation of the developing nephron. These organoids express markers of the (B) renal epithelium, including podocalyxin (PODXL; in magenta), lotus tetragonolobus lectin (LTL; in green), and e-cadherin (ECAD; in white), (C) endothelium, specifically, platelet endothelial cell adhesion molecule (CD31; in white), and (D) mesenchyme, including vimentin (VIM; in yellow) and Meis homeobox family (MEIS1/2/3; in red). This image was contributed by STEMCELL Technologies.
Materials
- Dulbecco’s phosphate-buffered saline without calcium and magnesium (D-PBS)
- Fixation solution: 4% paraformaldehyde (PFA) in D-PBS
- Permeabilization buffer: PBS-T, prepared fresh
- Dilute Triton™ X-100 in D-PBS for a final concentration of 0.2%
- Blocking buffer: 10% donkey serum in PBS-T, prepared fresh
- Mix thoroughly and store on ice or at 2 – 8°C while in use.
- Primary and secondary antibody/ies of choice
- Can be identified with the Antibody Dilution Finder
- 1 µg/mL DAPI in blocking buffer
Protocol
- Aspirate medium from the well containing kidney organoids. Carefully add 200 µL D-PBS to the well. Remove D-PBS.
- Fix: Add 85 µL 4% PFA in D-PBS to the well. Incubate at room temperature (15 – 25°C) for 15 minutes. Remove the solution.
- Wash the well 3X with 200 µL D-PBS. Remove D-PBS.Note: For later immunostaining, keep 200 µL D-PBS in the well, wrap plate with Parafilm®, and store at 2 – 8°C. Remove remaining D-PBS before proceeding to Step 4.
- Permeabilize: Add 200 µL PBS-T to the well. Incubate at room temperature (15 – 25°C) for 15 minutes. Remove PBS-T.
- Block: Add 200 µL 10% donkey serum in PBS-T to the well. Incubate at room temperature (15 – 25°C) for at least 30 minutes.
- Primary antibody staining: While incubating, prepare primary antibody solution by diluting each primary antibody in the blocking buffer. Remove blocking buffer from the well and add 80 µL primary antibody solution.
- Incubate at 2 – 8°C overnight with low shaking.
- Primary antibody wash: Add 200 µL D-PBS to the well and incubate at room temperature (15 – 25°C) for 5 minutes. Remove D-PBS. Repeat this wash step 5X for a total of 6 washes.
- Prepare secondary antibody solution by diluting each secondary antibody in blocking buffer with 1 µg/mL DAPI.
- Secondary antibody staining: Add 80 µL secondary antibody solution to the well.
- Incubate in the dark at room temperature (15 – 25°C) overnight with low shaking.
- Secondary antibody wash: Remove secondary antibody solution. Add 200 µL D-PBS to the well and incubate at room temperature (15 – 25°C) for 5 minutes. Remove D-PBS. Repeat this wash step 5X for a total of 6 washes.
- Add 200 µL D-PBS to stained cells; they are now ready for immunofluorescent imaging.Note: If not used immediately for imaging, wrap plate with Parafilm® and store in the dark at 2 – 8°C.
Notes
Safety Note
Prior to handling any chemical, consult the Safety Data Sheet (SDS) for proper handling and safety precautions.
References
- STEMCELL.com (Accessed 2024)
Performing Immunocytochemical Staining of PSC-Derived Kidney Organoids.





