Schiff’s reagent is a very sensitive means of detecting aldehydes, and can be used in a method to demonstrate deoxyribonucleic acid (DNA) specifically, in contrast to unstained ribonucleic acid (RNA). This method is the nucleal reaction of Feulgen and Rossenbeck, usually simply called the Feulgen stain or reaction. It is usually done with pararosaniline Schiff solution, but it works well with some others, including the fluorescent acriflavine solution.
Introduction
The technique involves treating sections with dilute hydrochloric acid. This treatment hydrolysis DNA and removes the bases. The sugar remains and reacts as an aldehyde, including condensation with Schiff’s reagent when it is subsequently applied. The typical red coloration where DNA was present is thus formed. The diagrams below illustrate the removal of the bases and the subsequent availability of an aldehyde, with the relevant parts in red.
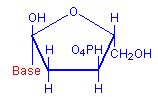
Deoxyribose with base
+
H2O
=
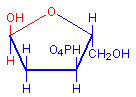
Deoxyribose hemiacetal
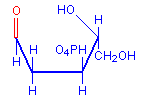
Deoxyribose aldehyde
In the original method the hydrolysis is done with 1N-hydrochloric acid at 60°C for an appropriate length of time, varying according to the fixative used. Strongly acid fixatives should be avoided since they may hydrolyze DNA during fixation, perhaps to the extent that all DNA is removed and there is no staining. Bouin’s picric acid, acetic acid, and formalin mixture is known to do this, although sometimes DNA can be demonstrated if the acid hydrolysis step is omitted during staining. Regardless of the fixative used, it is recommended that trials be conducted so the optimal hydrolysis time can be determined. The following may act as a guide:
Fixative Considerations
In the original method, the hydrolysis is done with 1N-hydrochloric acid at 60°C for an appropriate length of time, varying according to the fixative used. Strongly acid fixatives should be avoided since they may hydrolyze DNA during fixation, perhaps to the extent that all DNA is removed and there is no staining. Bouin’s picric acid, acetic acid, and formalin mixture is known to do this, although sometimes DNA can be demonstrated if the acid hydrolysis step is omitted during staining. Regardless of the fixative used, it is recommended that trials be conducted so the optimal hydrolysis time can be determined. The following may act as a guide:
| Fixative | Time | Comments |
|---|---|---|
| Carnoy | 8 minutes | – |
| Formalin | 8 minutes | NBF, formal saline, etc. |
| Formaldehyde vapor | 30-60 minutes | On freeze-dried tissue. |
| Formal sublimate | 8 minutes | B5 has a very similar formula. |
| Zenker | 5 minutes | – |
| Zenker-formal | 5 minutes | Zenker-formal and Helly are different. |
| Helly | 8 minutes | – |
| SUSA | 18 minutes | – |
| Bouin | Not recommended | Refers to Bouin’s picric-acetic-formalin. |
| Glutaraldehyde | Not recommended | Precede hydrolysis with an aldehyde block. |
It has been shown that the 60°C temperature used in the original 1N-hydrochloric acid hydrolysis can result in loss of stainable material, and it is recommended that 5N-hydrochloric acid at room temperature for 30 minutes or longer be used. This allows for less precise timing and darker staining. Pearse gives some times as a guide:
| Fixative | Time | Comments |
|---|---|---|
| Alcoholic fixatives | 20 minutes to 2 hours | – |
| Formalin fixatives | 35 minutes to 4 hours | – |
| Formaldehyde vapor | 2 hours to 8 hours | Freeze-dried tissue |
References
- Pearse, A. G. E., (1968, 1972)
Histochemistry: Theoretical and Applied, Ed. 3
Churchill Livingstone, Edinburgh, London, UK - Kiernan. J.A., (1999)
Histological and histochemical methods: Theory and Practice, Ed. 3
Butterworth Heinemann, Oxford, UK.






