Schiff’s reagent is a solution that will combine chemically with aldehydes to form a bright red product. In some, usually older, texts you will find Schiff’s reagent referred to as leucofuchsin. The prefix leuco means white and refers to the loss of color in the solution. However, a leucobase proper is produced by reduction, and its color is restored by oxidation. Schiff’s reagent does not have the original pararosanilin color restored. Rather, a new-colored compound is produced by the chemical combination with an aldehyde. For that reason, it is usual now to refer to it as Schiff’s reagent or fuchsin-sulfurous acid.
Introduction
It seems to make little difference as to the source of the sulfurous acid. As this is simply sulfur dioxide dissolved in water there are several ways to obtain it. The four procedures that have been recommended are:
- Sulfur dioxide gas from a cylinder is bubbled slowly through a solution of pararosanilin until it starts to change color. The container is then tightly stoppered and left in a dark place, usually overnight or longer.
- The sulfurous acid solution is purchased already made. Pararosanilin is dissolved in it without heat. The container is then tightly stoppered and left in a dark place, usually overnight or longer.
- Sodium or potassium metabisulphite or sulfite (commercial samples of which are apparently composed mainly of metabisulphite) are added to a pararosanilin solution. Hydrochloric acid is added, producing sulfur dioxide in the solution. The container is then tightly stoppered and left in a dark place, usually overnight or longer.
- Chemicals that decompose in solution to produce sulfur dioxides, such as sodium hydrosulfite (dithionite) or thionyl chloride are added to a solution of pararosanilin. The container is then tightly stoppered and left in a dark place, usually overnight or longer.
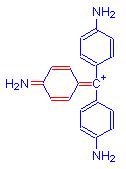
+ H2SO3 =
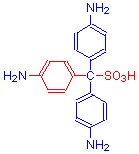
Note the loss of the quinoid ring of pararosanilin in the left diagram (highlighted in red) by the change from double to single bonds because of the addition of -SO3H and the formation of Schiff’s reagent.
Appearance
Schiff’s reagent should be colorless or very pale yellow. However, pararosanilin can contain other dyes, particularly if it is from a sample of basic fuchsin, which is a mixture. The other dyes present are usually homologs of pararosanilin, such as rosaniline and magenta II, but produce deep amber solutions and may color aldehydes brownish red. These darker products can be removed by adding a small amount of activated charcoal powder, shaking the solution for about a minute, and filtering. Sometimes this treatment doesn’t work and the solution remains brown. Unfortunately, if enough charcoal is then added to completely remove all the brown discoloration, the resulting clear solution may only give very pale staining. Usually, this means that a sample of basic fuchsin was used which contains large amounts of one of the other dyes. If possible buy samples of basic fuchsin specified as suitable for Schiff’s reagent, or buy pararosanilin.
As previously mentioned, Schiff’s reagent combines with aldehydes to give a bright red product. Histologically, the aldehydes are either attached to or produced from, a tissue structure. Therefore the tissue structure itself becomes colored bright red. The mechanism is the same for all aldehydes in tissues. The aldehyde condenses with Schiff’s reagent to make a new compound attached to the tissue. In the process, the chromophore reforms, and the color is produced.
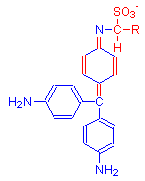
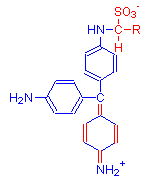
The new red product is shown with two formulas. Most explanations give the first as the final product. However, some texts present an alternative view incorporating one aldehyde. In either case, the dye is composed of Schiff’s reagent, the aldehyde and the chemical to which the aldehyde is attached. Kiernan reports that the latest investigations indicate the product is different from that given above and exists as a tautomer of the two products below.

+
2(R-CHO)
=
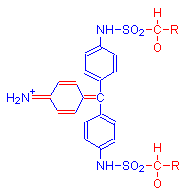
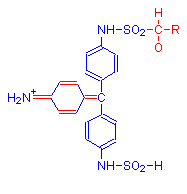
The single bonds of Schiff’s reagent shown in above reaction in the leftmost diagram (highlighted in red), reform into a quinoid ring in the product shown in the middle diagram. This is due to the addition of aldehyde. The formula on the right is a possible alternative product.
Pararosanilin Alternatives
Dyes other than pararosanilin can be used to make Schiff-type reagents. These are often referred to as pseudo-Schiff reagents. Usually, these dyes do not decolorize completely, although the color and translucency of the solution are often altered. It is possible that residual colored compounds in these solutions can stain tissues ionically. In order to remove this staining, it is customary to treat sections with acid alcohol afterward. Any staining left after acid alcohol treatment is a positive reaction from the pseudo-Schiff reagent.
None of these pseudo-Schiff’s reagents have gained anything approaching the popularity of pararosanilin Schiff’s reagent, and this solution remains the standard. The reason is the bright and clear red staining by pararosanilin Shiff’s reagent. The other dyes give inferior coloration, although a few are reasonably effective. The solutions that have gained the most favor are those made with fluorescent dyes, acriflavine (see below), for example. These enable a fluorescent positive result which can be very useful for demonstrating materials such as fungi, for instance, which may be present in small amounts.
Culling gives a list of many of these dyes, a few of which are listed here. For more information refer to Kasten’s papers below, quoted by Culling.
| Dye | CI Number | Color |
|---|---|---|
| Acid fuchsin | 42685 | Violet |
| Acriflavine | 46000 | Yellow |
| Azure A | 52005 | Blue |
| Azure C | 52005 | Blue |
| Crystal violet | 42555 | Blue-violet |
| Methyl violet | 42535 | Violet |
| Methylene blue | 52015 | Blue |
| Safranin O | 50240 | Red |
| Thionin | 52005 | Blue |
| Toluidine blue | 52040 | Blue |
Sulphite Rinses
When tissues are removed from Schiff’s reagent and washed, there is inevitably some carryover to the washing fluid. If tap water is used there is a rapid recoloring of the reagent and the water turns quite red. Initially, there were some concerns that this recolored Schiff’s reagent would behave as a basic dye and stain the tissues, giving false positives. For that reason, sulfite rinses were recommended. These are dilute sulfurous acids and they are used to wash off the Schiff’s reagent for a few minutes, diluting it out well enough before washing the tissues in water, that there is no likelihood of non-aldehyde staining. Experience over many years has shown that these rinses are not necessary and washing well with tap water is satisfactory, provided the sections do not stay in recolored Schiff’s reagent.
Example Sulphite Rinse Formulation
| Material | Amount | |
|---|---|---|
| Water | 90 | mL |
| Potassium metabisulphite 10% aqueous | 5 | mL |
| N1 hydrochloric acid | 5 | mL |
If a pseudo-Schiff’s reagent is used, then the sections should be treated with 1% hydrochloric acid in 70% ethanol for 5-10 minutes after rinsing it off. Some of these dyes may not be completely decolorized and may stain ionically. The acid alcohol will remove any such staining. After the acid alcohol treatment, the sections should be washed with tap water as usual.
Aldehyde Detection
Although Schiff’s reagent is used to detect aldehydes, these are not usually found free in tissues and must be produced in some fashion. There are three ways this can be done. The first, and by far the commonest, is to oxidize certain of the tissue components. This produces aldehyde groups, enabling the material to which the component is attached to be demonstrated. The second way is to treat sections with acids to convert some of the deoxyribose in DNA to aldehydes and then color them with Schiff’s reagent. The third is to attach an aldehyde directly to the tissues, usually a protein, then demonstrate the aldehyde with Schiff’s reagent.
This latter can be seen easily with glutaraldehyde fixation for light microscopy. Glutaraldehyde has two aldehyde groups and, if the molecule attaches to the tissue by one of them, the other remains unattached when fixation is complete. Due to this, with any procedure using Schiff’s reagent the unattached aldehydes will react with it to produce a deep pink background. So, before Schiff’s reagent can be applied to these sections, the aldehydes should be blocked so they cannot react. In practice, it is usually convenient to apply an aldehyde block immediately after dewaxing and bringing the sections to water, washing well, then continuing with the staining method, including any oxidation required. In this way staining from the glutaraldehyde is eliminated.
In any critical application using glutaraldehyde fixation, two controls must be used. The first is a section in which any aldehyde present has been inhibited from staining by the application of an aldehyde block. The second is a section in which Schiff’s reagent has been applied to the tissue without any pretreatment. The first section thus blocks pre-existing aldehydes, while the second identifies where they were located. In addition, of course, known negative and positive control sections for the target tissue element should be employed. If these two latter controls do not stain as expected, then the Schiff’s reagent, and any others, should be examined to ensure they have not deteriorated and are suitable for use.
References
- Pearse, A. G. E., (1968, 1972)
Histochemistry: Theoretical and Applied, Ed. 3
Churchill Livingstone, Edinburgh, London, UK - Kiernan. J.A., (1999)
Histological and histochemical methods: Theory and Practice, Ed. 3
Butterworth Heinemann, Oxford, UK. - Culling C.F.A., (1974)
Handbook of histopathological and histochemical techniques Ed. 3
Butterworth, London, UK.
Citing:
Kasten, F.H., (1958), Stain Technology, vol.33, pp.39
Kasten, F.H., (1959), Histochemie, vol.1, pp.466 - Lillie, R.D., (1954)
Histopathologic technique and practical histochemistry Ed.2
Blakiston, New York, USA.