Lynch & Inwood's Gold
for Amyloid
Materials
- Chloro-auric acid, 1% aqueous
- Hydrogen peroxide, 3% aqueous
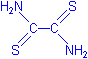
- Iodine solution
Material Amount Iodine 1 g Potassium iodide 2 g Distilled water 100 mL Mix the potassium iodide and iodine crystals together. Add 5 mL of the water and mix until both have dissolved. Add the rest of the water.
Tissue Sample
5-10µ sections from formalin fixed, paraffin embedded tissues, mounted on slides are suitable.
Protocol
- Bring sections to water through xylene and ethanols.
- Place in the iodine solution for 2½-5 minutes.
- Rinse with distilled water, 3 times of 5-10 seconds each.
- Place in chloro-auric acid solution for 2½-5 minutes.
- Rinse with distilled water, 3 times of 5-10 seconds each.
- Place in fresh 3% hydrogen peroxide at 37°C for 6-36 hours.
- Dehydrate with ethanol, clear with xylene and mount using a resinous medium.
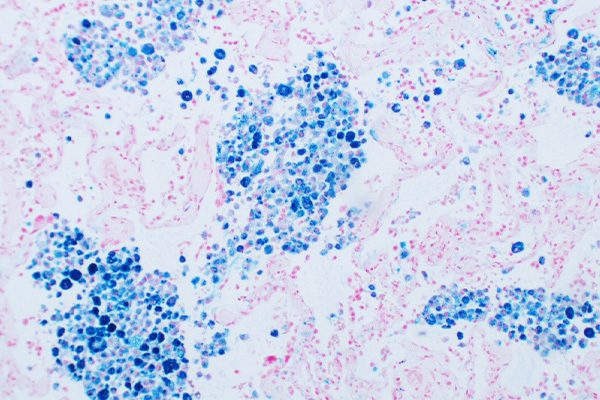
Expected Results
- Amyloid – golden yellow to faint purple.
- Erythrocytes, muscle and liver – blue
- Nuclei and cytoplasm – brown
- Collagen – grey
Safety Note
Prior to handling any chemical, consult the Safety Data Sheet (SDS) for proper handling and safety precautions.
References
- Lynch, M.J. and Inwood, M.J.H., (1963),
Gold as a permanent stain for amyloid,
Stain Technology, v 38, page 260.