Musto's Hematoxylin Scarlet-Saffron
for Elastic
Materials
Stock hematoxylin
| Material | Amount | |
|---|---|---|
| Hematoxylin | 2 | g |
| Ethanol, 95% | 100 | mL |
Stock ferric chloride
| Material | Amount | |
|---|---|---|
| Ferric chloride, hexahydrate | 12.4 | g |
| Distilled water | 500 | mL |
| Hydrochloric acid, concentrated | 5 | mL |
Stock iodine
| Material | Amount | |
|---|---|---|
| Potassium iodide | 4 | g |
| Iodine | 2 | g |
| Distilled water | 100 | mL |
Working solution
| Material | Amount | |
|---|---|---|
| Stock hematoxylin | 30 | mL |
| Stock ferric chloride | 20 | mL |
| Stock iodine | 10 | mL |
Tungstophosphoric acid
| Material | Amount | |
|---|---|---|
| Tungstophosphoric acid | 5 | g |
| Distilled water | 100 | mL |
Acetic acid
| Material | Amount | |
|---|---|---|
| Acetic acid, glacial | 1 | mL |
| Distilled water | 99 | mL |
Woodstain scarlet
| Material | Amount | |
|---|---|---|
| Woodstain scarlet, 1% aqu. | 50 | mL |
| Tungstophosphoric acid | 2 | g |
Prepare fresh.
Alcoholic saffron solution
| Material | Amount | |
|---|---|---|
| Saffron | 1 | g |
| Ethanol, absolute | 100 | mL |
Extract the saffron in ethanol at 56°C for 24 hours, shaking occasionally. Cool and filter.
Tissue Sample
A 10% Formalin variant is suitable. Paraffin sections at 5µ are preferred.
Protocol
- Bring sections to water via xylene and ethanol.
- Place sections in Bouin’s fluid at 60°C for 1 hour.
- Remove the yellow colouration with running tap water.
- Place in working solution for 45 minutes.
- Wash well with tap water, then rinse with distilled water.
- Place into the woodstain scarlet solution for 5 minutes.
- Rinse well with distilled water.
- Differentiate in tungstophosphoric acid solution for 5 minutes.
- Transfer directly to acetic acid solution for 5 minutes.
- Dehydrate with 95% ethanol for 1 minute.
- Place in absolute ethanol, two changes, for 1 minute each.
- Place in alcoholic saffron solution for 5-10 minutes.
- Place in absolute ethanol, two changes, for 1 minute each.
- Clear with xylene and mount with a resinous medium
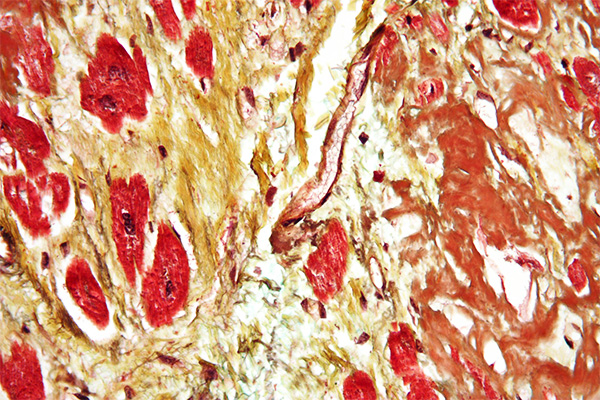
Expected Results
- Nuclei – black
- Elastic fibres – black
- Muscle – pale red
- Collagen – yellow
Notes
- The original paper also gave a procedure using a Van Gieson counterstain.
- This is a modification of Verhöeff’s hematoxylin which does not require differentiating.
Safety Note
Prior to handling any chemical, consult the Safety Data Sheet (SDS) for proper handling and safety precautions.
References
- Musto, Linda, (1981),
Improved iron-hematoxylin stain for elastic fibers.,
Stain Technology, v 56, page 185-7.