Periodic Acid Schiff Diastase
The periodic acid Schiff reaction (PAS) is used to demonstrate the presence of 1-2-glycols, including glycogen. Sometimes it is necessary for the glycogen to be removed so that a clearer picture of non-glycogen carbohydrates may be seen. Glycogen may be removed with amylase (diastase).
Materials
- Hog α-amylase, 0.1% in 0.02M phosphate buffer, pH 6.0
- Periodic acid – 1% aqueous
- Schiff’s reagent
- Mayer’s hemalum
Tissue Sample
5µ paraffin sections of neutral buffered formalin fixed tissue are suitable. Other fixatives are usually satisfactory, although glutaraldehyde should be avoided.
Protocol
- Two consecutive test and 2 consecutive control sections.
- Bring sections to water via xylene and ethanol.
- Place one test section and one known positive section into water.
- Place the other test section and the other known positive in the amylase solution for 30-60 minutes at 35-45°C
- Wash both digested sections with tap water
- Celloidinise the sections if desired.
- Place into periodic acid for 10-30 minutes.
- Rinse well with tap water.
- Rinse with distilled water.
- Place in Schiff’s reagent for 10-30 minutes.
- Wash off with distilled water.
- Wash well with tap water for about 10 minutes.
- Counterstain with Mayer’s hemalum for 2 minutes.
- Wash well with tap water until hemalum is blued.
- Dehydrate with ethanol, clear with xylene and coverslip using a resinous medium.
Expected Results
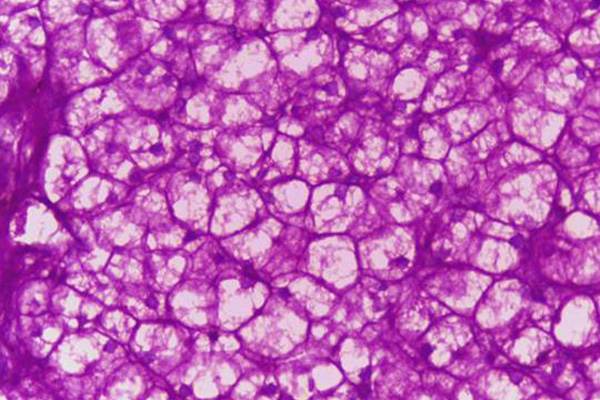
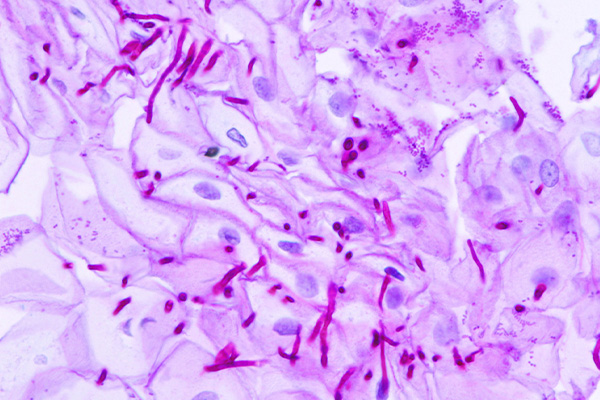
- Glycogen – red in the undigested section, absent from the digested section.
- Oxidisable carbohydrates – red
- Nuclei – blue
Notes
- If the intent is to remove glycogen rather than identify it, a more convenient approach in a routine service laboratory is to dissolve the amount of α-amylase that would cover a 1 cm circle about 1 mm deep in 15 mL (1 test tube) of distilled water. Shake for a few minutes, then filter onto the sections and digest at room temperature. Glycogen is usually removed within 30 minutes to 1 hour. Wash well and continue with a regular PAS (step 7).
- Please note that an older variation of the procedure in Note 1 was to collect saliva and use that for the digestion. This is strongly deprecated. Human body fluids may contain transmissible bacterial and viral contaminants, so anyone handling such a slide will be at risk.
- Hog α-amylase is recommended as being consistent and effective. Purchase a product with a high α-amylase activity.
- Glutaraldehyde fixation leaves free aldehyde groups attached to tissues, which causes an overall positive reaction. These groups may be stopped from reacting with an appropriate procedure such as the aniline-acetic aldehyde block.
- The tap water wash at step 7 is necessary to develop the red color. Within limits, the longer the wash the darker the color.
- Originally, it was recommended that the Schiff’s reagent be washed off with dilute sulfurous acid (the sulfite rinses). Since water recolors Schiff’s reagent, it was believed that a water wash could lead to false positive results. It is now known this is not the case, provided the Schiff’s reagent is removed quickly and the sections do not stay in water contaminated with it for extended periods.
Safety Note
Prior to handling any chemical, consult the Safety Data Sheet (SDS) for proper handling and safety precautions.