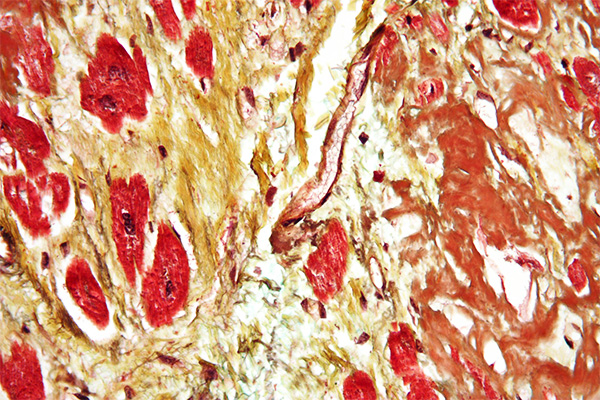
Paquin & Goddard's Trichrome
for Elastic and Collagen
Materials
- Paquin & Goddard’s iron hematoxylin or equivalent
- Solution A
Material Amount Eosin Y 0.7 g Phloxine 0.3 g Orange G 0.1 g Phosphotungstic acid 1 g Distilled water 1 L - Solution B
Material Amount Phosphotungstic acid 2 g Distilled water 1 L - Solution C
Material Amount Acetic acid, glacial 4 mL Distilled water 1 L - Solution D
Material Amount Aniline blue 0.4 g Acetic acid, glacial 10 mL Distilled water 990 mL
Tissue Sample
5µ paraffin sections of Masson’s picric-chrome-formalin fixation was specified. Most trichrome stains benefit from picric acid or mercuric chloride fixation. The specified fixation may be compensated for by secondary fixation of sections in Bouin’s fluid.
Masson’s picric-chrome-formalin contains:
| Material | Amount | |
|---|---|---|
| Picric acid, sat. aqu. | 187.5 | mL |
| Chromium aluminum sulphate | 7.5 | g |
| Formalin, Conc. 40% | 67.5 | mL |
- Add the alum to the formalin and leave for 1 hour.
- Add the picric acid solution.
- Let stand for 24 hours, and filter.
- Return to tissue sample.
Protocol
- Bring sections to water via xylene and ethanol.
- Stain with Paquin & Goddard’s iron hematoxylin.
- Wash with water.
- Place into solution A for 5 minutes.
- Place into solution B for 5 minutes.
- Rinse twice with solution C.
- Place into solution D for 5 minutes.
- Rinse twice with solution C.
- Place into solution B for 5 minutes.
- Place into solution C for 30 minutes.
- Rinse quickly with 95% ethanol.
- Dehydrate with iso-amyl alcohol.
- Clear with toluene and mount with a resinous medium.
Expected Results
- Nuclei – black
- Elastic – red
- Cytoplasm – pink
- Collagen – blue
Notes
- If iso-amyl alcohol is not available, t-butanol is often suitable when the staining is likely to be removed with ethanol, although rapid dehydration with ethanol can often overcome this.
- Although toluene was specified for clearing, xylene is likely suitable.
Safety Note
Prior to handling any chemical, consult the Safety Data Sheet (SDS) for proper handling and safety precautions.
References
- Gray, Peter. (1954)
The Microtomist’s Formulary and Guide.
Originally published by: The Blakiston Co.
Republished by: Robert E. Krieger Publishing Co.
Citing:
Paquin & Goddard, (1947)
Bulletin of the International Association of Medical Museums and
Journal of Technical Methods, v.27, pp.198